14+ li orbital diagram
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere pedosphere geosphere hydrosphere and atmosphere of the EarthCarbon is the main component of biological compounds as well as a major component of many minerals such as limestoneAlong with the nitrogen cycle and the water cycle the carbon cycle comprises a. Li Z 3.

Luminescent And Photofunctional Transition Metal Complexes From Molecular Design To Diagnostic And Therapeutic Applications Journal Of The American Chemical Society
It is named after the Roman god Mercurius god of commerce messenger of the gods and mediator between gods and mortals corresponding to the Greek god Hermes Ἑρμῆς.
. The terms atomic orbital and molecular orbital were introduced by Robert S. Ultrasound-induced gas bubbles in tissue can temporarily minimize optical scattering enabling laser light to be focused at greater depth for higher-resolution imaging. Carbon dioxide chemical formula CO 2 is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms.
This is called quantum jump. The Moon is the fifth largest satellite in the Solar SystemIt is larger than any of the known dwarf planets and is the largest and most massive satellite relative to its parent planet. A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular.
The Moon is Earths only natural satelliteIts diameter is about one-quarter the diameter of the Earth comparable to the width of Australia. Preparing for the next pandemic and what the future holds for science in China. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same.
Space-based solar power SBSP SSP is the concept of collecting solar power in outer space by solar power satellites SPS and distributing it to EarthIts advantages include a higher collection of energy due to the lack of reflection and absorption by the atmosphere the possibility of no or very little night and a better ability to orient to face the sun. Mercury is the smallest planet in the Solar System and the closest to the SunIts orbit around the Sun takes 8797 Earth days the shortest of all the Suns planets. For example If n 1 n 1.
Particle physics which has revealed how matter behaves at the smallest scales. Lithium is the element of group. Curiosity is a car-sized Mars rover designed to explore the Gale crater on Mars as part of NASAs Mars Science Laboratory MSL mission.
Must contain at least 4 different symbols. Atmospheric entry is the movement of an object from outer space into and through the gases of an atmosphere of a planet dwarf planet or natural satelliteThere are two main types of atmospheric entry. Mulliken in 1932 to mean.
There are 14 s-block elements in the 118 elements of the periodic table. We already know that the p-subshell has three orbitals. 10 atm 79 nitrogen 21 oxygen.
For light atoms the spinorbit interaction or coupling is small so that the total orbital angular momentum L and total spin S are good quantum numbersThe interaction between L and S is known as LS coupling RussellSaunders coupling named after Henry Norris Russell and Frederick Albert Saunders who described this in 1925 or spin-orbit. The fourth electron fills this orbital. Orbital number of the subshell.
2 6 10 14. It is found in the gas state at room temperature. Alkali metals react with dry hydrogen at 400C to form metallic hydride compounds.
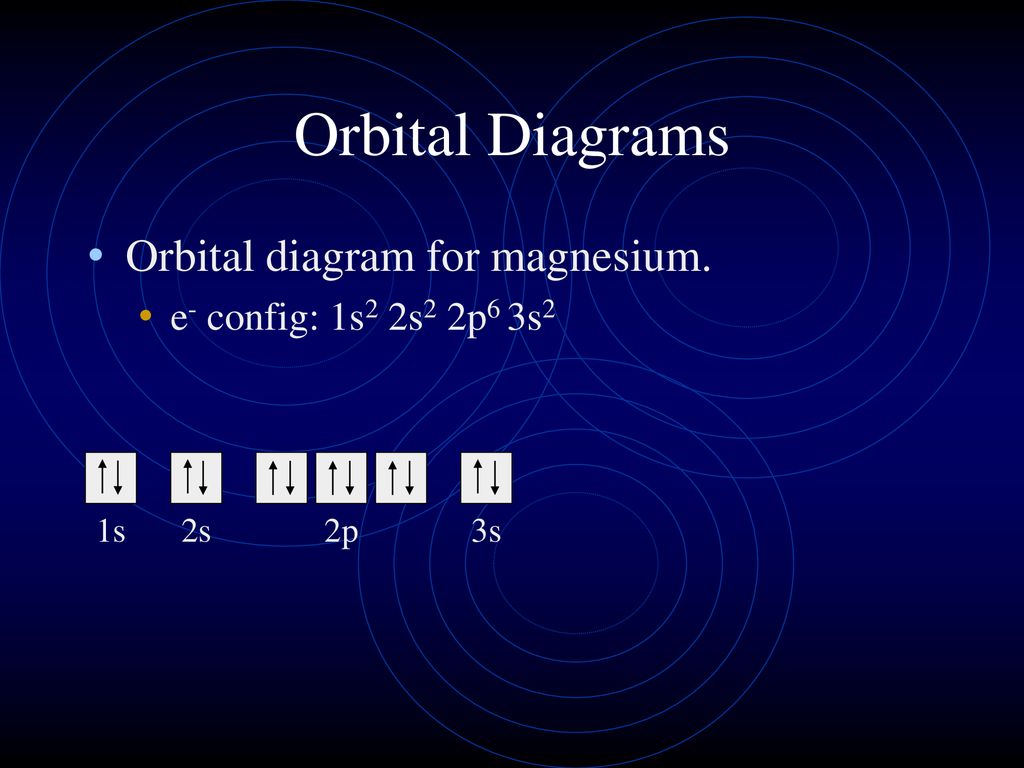
The atomic nucleus is the small dense region consisting of protons and neutrons at the center of an atom discovered in 1911 by Ernest Rutherford based on the 1909 GeigerMarsden gold foil experimentAfter the discovery of the neutron in 1932 models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Orbital diagram for titaniumTi Titanium excited state electron configuration. While the future cannot be predicted with certainty present understanding in various scientific fields allows for the prediction of some far-future events if only in the broadest outline.
Atoms can jump from one orbital to another orbital in the excited state. 4s 2 4p 6 4d 10 4f 14. Like Venus Mercury orbits the Sun within.
Which has been discussed in detail above. The elements in group-1 of the periodic table are alkali metals. In the air carbon dioxide is transparent to visible light but absorbs infrared radiation acting as a greenhouse gasIt is a trace gas in Earths atmosphere at 417.
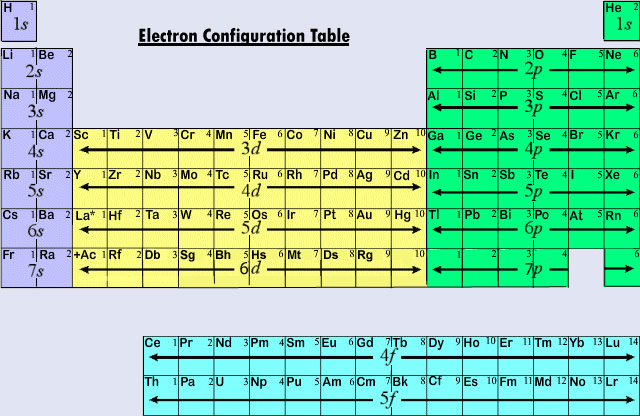
The search form recognizes IAU numbers designations names and JPL SPK-ID numbers. Although more generally the rule is applicable for the s-block and p. Predict the electron configuration for calcium.
These fields include astrophysics which studies how planets and stars form interact and die. Curiosity was launched from Cape Canaveral CCAFS on 26 November 2011 at 150200 UTC and landed on Aeolis Palus inside Gale crater on Mars on 6 August 2012 051757 UTC. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atoms nucleusThe term atomic orbital may also refer to the physical region or space where the electron can be.
The ground state electron configuration of titanium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2. Lithium is an alkali metal. Li Na K Cs all the elements of group-1 react with hydrogen to.
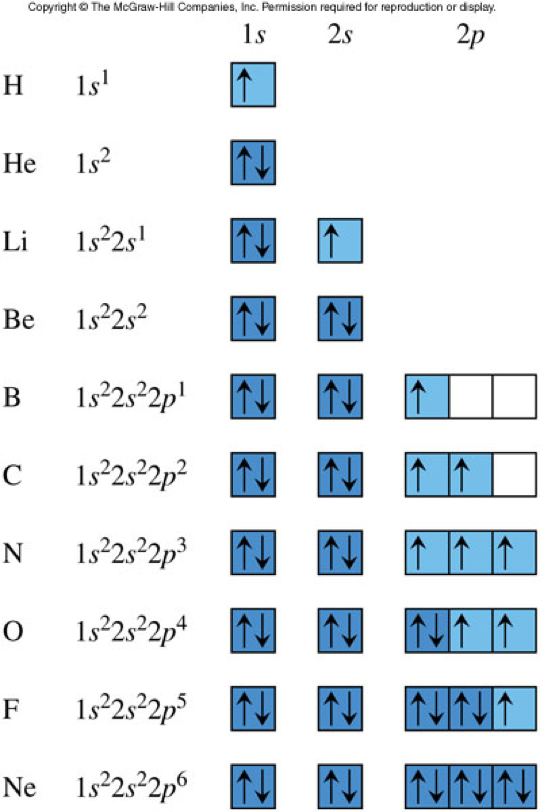
The ground-state electron configuration of fluorine is 1s 2 2s 2 2p 5. We already know that the p-subshell has three orbitals. Aye-ayes use their long skinny middle fingers to pick their noses and eat the mucus.
When searching for a particular asteroid or comet it is best to use either the IAU number as in 433 for asteroid 433 Eros or the primary designation as in 1998 SF36 for asteroid 25143 1998 SF36However using the asteroidcomet name will also work as in Ceres for. The electron configuration of all the elements can be done through the orbital diagram. The orbitals are p x p y and p z and each orbital can have a maximum of two electrons.
ASCII characters only characters found on a standard US keyboard. 1013 kPa 147 psi. Atoms can jump from one orbital to another orbital in an excited state.
Orbital boosting can be performed by the stations two main engines on the Zvezda service module or Russian or European spacecraft docked to Zvezda s aft port. In atomic theory and quantum mechanics an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. The Moon is a planetary-mass object that formed a.
Term symbols with LS coupling. And controlled entry or reentry of a spacecraft capable of being navigated or. 6 to 30 characters long.
Uncontrolled entry such as the entry of astronomical objects space debris or bolides. Finally the 14 columns at the bottom of the table correspond to the filling of the seven orbitals in an f subshell. Paul Villard a French chemist and physicist discovered gamma radiation in 1900 while studying radiation emitted from radiumVillard knew that his described.
1s 2 2s 1. To write the orbital diagram of lithiumLi you have to do the electron configuration of lithium. Diagram structure of International Space Station after installation of solar arrays as of September 2021.
The first gamma ray source to be discovered was the radioactive decay process called gamma decayIn this type of decay an excited nucleus emits a gamma ray almost immediately upon formation. The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell giving it the same electronic configuration as a noble gasThe rule is especially applicable to carbon nitrogen oxygen and the halogens. This is called quantum jump.
The Bradbury Landing site was less than 24 km 15 mi from. In chemistry a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a moleculeThis function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The third electron goes into the next orbital in the energy diagram the 2s orbital.
Molecular Orbital Theory Mot Chemistry Study Material Emedicalprep Com Emedicalprep
Chem4kids Com Lithium Orbital And Bonding Info
Lecture 7 Presentation

Lithium Li

Oneclass Which One Of The Following Is The Correct Orbital Diagram For Nitrogen An Accepted Abbrevi

Superatom In Superatom Rhh Ag24 Sphme2 18 2 Nanocluster Yi 2021 Angewandte Chemie International Edition Wiley Online Library

Bisaryl And Bisalkynyl Diruthenium Iii Iii Compounds Based On An Electron Deficient Building Block Inorganic Chemistry

Molecular Orbital Diagram Wikipedia

Energies Of The Occupied And Vacant Single Particle Orbitals Of W 19 Download Scientific Diagram
Electron Configuration

Lithium Orbital Diagram Electron Configuration And Valence Electrons

Molecular Orbital Diagram Of Lithium Molecule Nature Of Chemical Bond Chemistry Class 11 Youtube
Orbital Diagrams Ppt Download
Two Point Resistances In The Generalized Phenylenes Springerlink
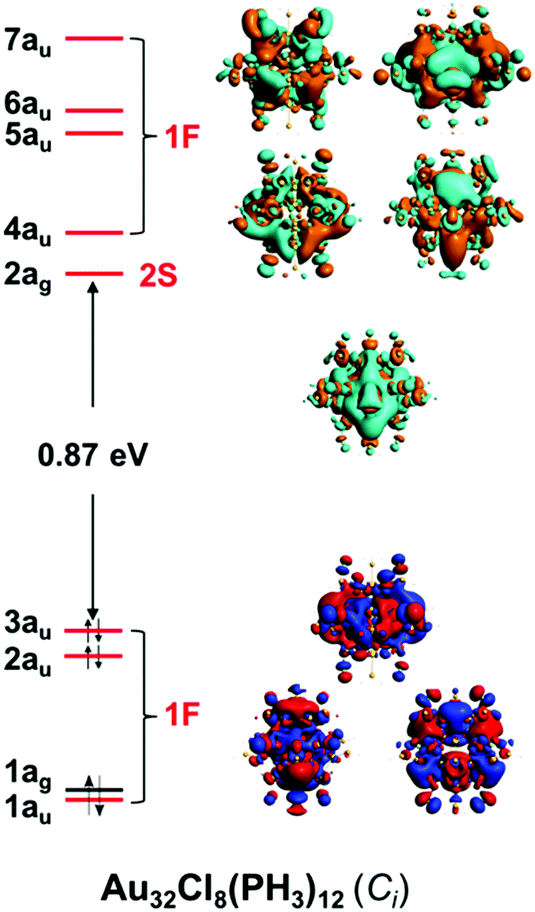
Electron Count And Electronic Structure Of Bare Icosahedral Au 32 And Au 33 Ionic Nanoclusters And Ligated Derivatives Stable Models With Intermediat Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 D0cp03735d

Chapter 8 Electron Configuration And Chemical Periodicity Video Solutions Chemistry The Molecular Nature Of Matter And Change Numerade
Solved Write Orbital Diagrams For The Following Atoms 15p19k28ni35br58ce Course Hero